Abstract
Introduction and objectives:
During an adaptive immune response antigen-specific T cells rapidly proliferate and differentiate into cytotoxic T lymphocytes. Most of these cells undergo apoptosis but some develop into high-affinity memory CD8+ T cells. The BCL-2 family of proteins regulates apoptosis and has a critical role in development and maintenance of the immune system. Venetoclax (Venclexta™, ABT-199) is a selective BCL-2 inhibitor that increases tumor cell apoptosis, and is approved by the FDA for patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL), with or without 17p deletion, who have received at least one prior therapy. Given the critical role of BCL-2 in the regulation of the immune system, we hypothesized that venetoclax may affect the anti-tumor activity of immune checkpoint inhibitors.
Results:
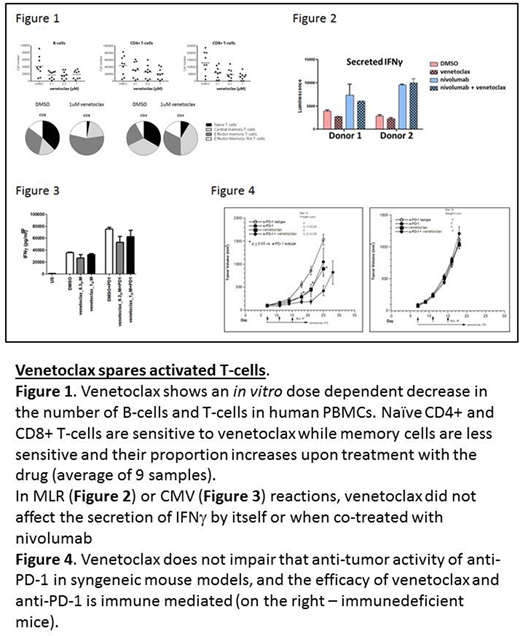
To interrogate the effects of venetoclax on T cells we initially performed a series of in vitro studies using human lymphocytes treated with clinically relevant doses of the drug. As previously reported (Khaw et al., Leukemia; 28(6):1207-1215, 2014), human peripheral blood mononuclear cells (PBMCs) treated with venetoclax exhibited a dose-dependent decrease in the number of B-cells and T-cells (CD4+ and CD8+ T-cells). Upon further characterization of the surviving T cells, we found that while the proportion of naïve T-cells decreased with increasing venetoclax concentrations, the proportion of memory T-cells increased, specifically CD8+ and CD4+ T effector memory cells (Figure 1).
We next examined the effects of venetoclax on T-cell function in vitro in response to immune stimulation with or without immune checkpoint blockade. To address this we performed a mixed lymphocyte reaction (MLR) assay, in which primary monocyte-derived dendritic cells from one donor were cultured with CD4+ T-cells from another donor. In the MLR reaction we observed that venetoclax reduced CD4+ T-cell viability in a dose-dependent manner, but it did not limit T-cell proliferation of surviving cells. Venetoclax did not affect IFNγ secretion within these surviving cells and, more importantly, did not reduce the effects of the checkpoint inhibitor nivolumab (Figure 2).
To test the effects of venetoclax on antigen-specific T cells, we performed a cytomegalovirus (CMV) recall assay where PBMCs from CMV-positive human subjects were incubated with CMV antigen and the activity of T cells was measured by IFNg secretion. Although venetoclax treatment reduced the total number of cells, IFNg production from antigen-specific CMV+ T cells remained comparable to DMSO control and combining venetoclax with nivolumab did not affect the anti-PD-1 response (Figure 3).
Finally, to investigate the effects of venetoclax in combination with anti-PD-1 therapy in vivo we used the murine syngeneic tumor model MC38. Venetoclax did not impair the efficacy of anti-PD-1, and in some studies increased efficacy relative to either anti-PD-1 or venetoclax monotherapy alone. To determine whether the efficacy of the venetoclax-anti-PD-1 combination is immune-mediated, we transplanted immunodeficient mice with MC38 cells and repeated the same treatment regimens. The lack of efficacy in any of the treatment arms indicates that the contribution of venetoclax to efficacy in this solid tumor model is immune-mediated (Figure 4).
Conclusions:
These data suggest that venetoclax treatment results in loss of naïve but not memory T cells. Venetoclax did not affect the viability, the induction or frequency of memory T cells. In human in vitro experiments and in an in vivo syngeneic tumor model venetoclax did not antagonize the therapeutic effect of anti-PD-1. Contrary to our initial hypothesis, we find that modulation of the immune system by venetoclax may support its potential use for immune-based cancer therapy, as memory T-cells can rapidly acquire effector and cytotoxic function to eliminate cancer cells. Taken together, we provide evidence that venetoclax in combination with immune checkpoint inhibitors should be further explored as a therapy for cancer patients.
All authors are employees of AbbVie. The design, study conduct, and financial support for this research were provided by AbbVie. AbbVie and Genentech participated in the interpretation of data, review, and approval of the publication.
Mathew:AbbVie Inc.: Employment. Haribhai:AbbVie Inc.: Employment. Kohlhapp:AbbVie Inc.: Employment. Duggan:AbbVie Inc.: Employment. Ellis:AbbVie Inc.: Employment. Riehm:AbbVie Inc.: Employment. Robinson:AbbVie Inc.: Employment. Shi:AbbVie Inc.: Employment. Bhathena:AbbVie Inc.: Employment. Leverson:AbbVie Inc: Employment, Equity Ownership, Patents & Royalties. Pappano:AbbVie Inc.: Employment. Donawho:AbbVie Inc.: Employment. Uziel:AbbVie Inc.: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal